Computational Chemistry in the Classroom
chemical modeling; Diels–Alder; call for more labs
Chemistry has traditionally been taught with a combination of 2D drawings and 3D Molymod kits. 2D drawings are perfect for grasping the fundamentals of bonding and the rearrangements that happen during reactions, while Molymod kits offer a tactile way to experience things like stereochemistry and Bürgi–Dunitz attack angles. Despite being incredibly useful, these tools can’t provide direct feedback as to which stereoisomer has been built or whether the barrier to a reaction is too high, so using them well requires careful guidance. Sadly, most classes never introduce students to more advanced modeling and visualization tools, perhaps due to the difficulties of accessing and using such tools.
One of the things we’ve found at Rowan is how useful computational chemistry can be for explaining chemical concepts. With Rowan, students can easily convert between 2D and 3D representations to bring their drawings to life. They can rotate a 3D molecule to better understand stereochemistry. They can map out reactions and see how the molecules transform along the reaction path, and visualize the energy barrier to the reaction. They can gain an appreciation for steric hindrance by seeing how parts of a molecule clash and thus understand cyclohexane A-values or exit vectors in protein–ligand binding.
However, there is a dearth of good materials for learning with computational chemistry, as most curricula are overly focused on teaching the tool (e.g. selecting the optimal DFT functional and basis set, using the command line, submitting jobs to a compute cluster) and not enough on what the tool can teach us. To that end, we’ve been working on some lab materials to highlight how computation can be used to teach chemistry. Isaiah Sippel, one of our summer interns, developed our first such lab, which focuses on the Diels–Alder reaction.
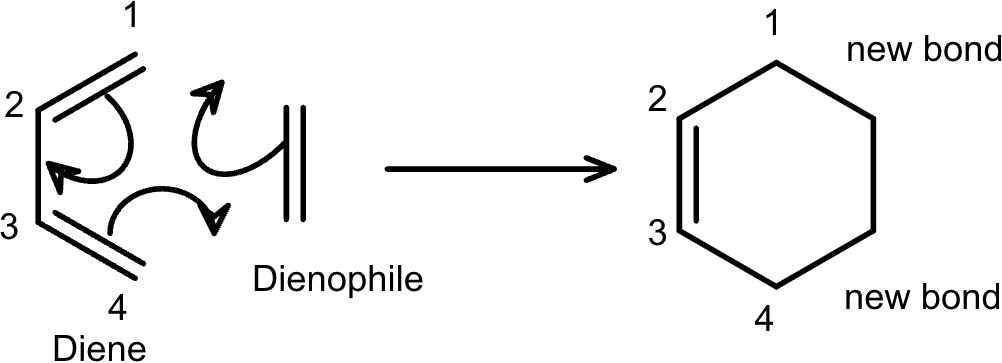
The Diels–Alder reaction is a straightforward [4+2] cycloaddition wherein three π bonds and a σ bond undergo a rearrangement to form two new σ bonds and one π bond. The rate of the reaction is affected by the various substituents on the diene and dienophile, and the rate can be tailored with careful application of electron-donating and electron-withdrawing groups.
Our new lab walks students through the Diels–Alder reaction, showing them how the bonds change over the course of the reaction and helping them visualize the formation of the new bonds. The lab has students determine the effect of various substituents on the activation barrier to determine trends, and then apply the insights to novel substituents. Finally, students are given a more complicated example and an open opportunity to play around with the chemistry to discover more for themselves.
The lab is designed to take around two hours for students who are completely new to the Diels–Alder reaction and computational chemistry, but sufficiently motivated students could finish it in closer to one hour. All of the materials are freely available and can be accessed in the labs section of Rowan’s documentation.
We hope to add more labs throughout the summer. If you are interested in collaborating on the development of new teaching materials or want to start using Rowan in your classroom, we’d love to hear from you at contact@rowansci.com.